Comprehensive validation and qualification services for aseptic processing and packaging systems
Explore the vital role of aseptic processing and packaging systems in ensuring product safety across the pharmaceutical and food industries. Learn about the importance of validation and qualification in maintaining sterile environments and preventing contamination. Thermosoft Technologies offers comprehensive solutions to uphold compliance with regulatory standards while enhancing operational efficiency. Partnering with experienced professionals can significantly reduce risks, improving product quality and consumer safety. Discover how rigorous validation processes can safeguard public health and contribute to the reliability of aseptic operations.
Our aseptic processing expertise helps you maintain product integrity while ensuring safety standards are met. We offer complete validation services for all types of aseptic systems.
Comprehensive audits of aseptic processing and packaging systems to identify potential issues and improvement areas.
Scientific assessment and establishment of sterilization processes with documented validation protocols.
Root cause analysis of spoilage issues, with actionable recommendations for corrective measures.

Expert consultation on evaluation and selection of equipment and packaging suppliers for your specific product needs.
Comprehensive audits for sterilization, filling and packaging systems to ensure regulatory compliance.
Comprehensive audits for sterilization, filling and packaging systems to ensure regulatory compliance.
Support for implementing new technologies with focus on regulatory acceptance and validation.
Thermosoft Technologies has established itself as a leading provider of validation and qualification services within the aseptic processing sector. The company operates with the mission of ensuring the highest standards of aseptic integrity for its clients. By offering comprehensive validation and qualification solutions, Thermosoft positions itself as a trusted partner for organizations looking to maintain compliance with stringent regulatory requirements in the food industry. The core principle of aseptic processing is to ensure that both the product and the packaging are sterilized separately and then combined in a way that maintains their sterility, effectively eliminating the risk of microbial contamination. Validation and Qualification Services for Aseptic processing and packaging, Sterilization evaluation and process establishment. We offer validation audits and spoilage investigations.


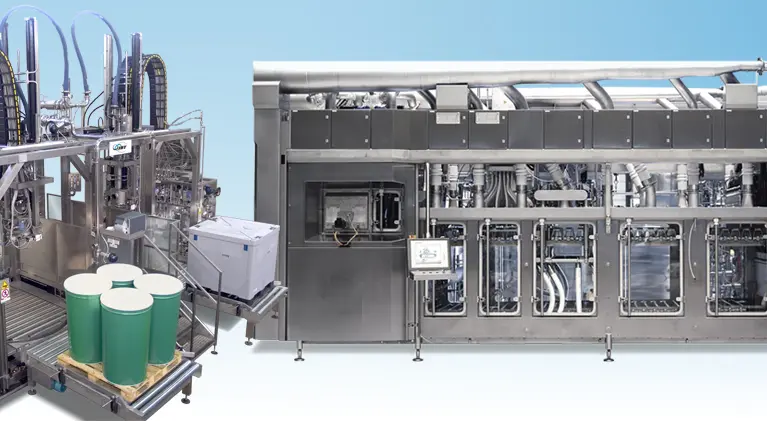




Thermosoft Technologies specializes in aseptic process authority, ensuring food and beverage safety through comprehensive validation services. Our expertise in sterilizer validation, aseptic tank checks, and compliance with FDA regulations like FSMA supports manufacturers in maintaining product integrity. We offer tailored solutions in process design and optimization, along with audits and training to navigate complex aseptic processes. Partner with us for reliable, efficient, and compliant processing that meets the highest standards.
In addition to our validation services, Thermosoft Technologies offers tailored solutions for process design and optimization, ensuring your facility maintains efficient operation without compromising safety. We provide audits, training, and troubleshooting support to assist clients in navigating complex aseptic processes. This comprehensive approach ensures that our clients meet both quality system requirements and regulatory standards.
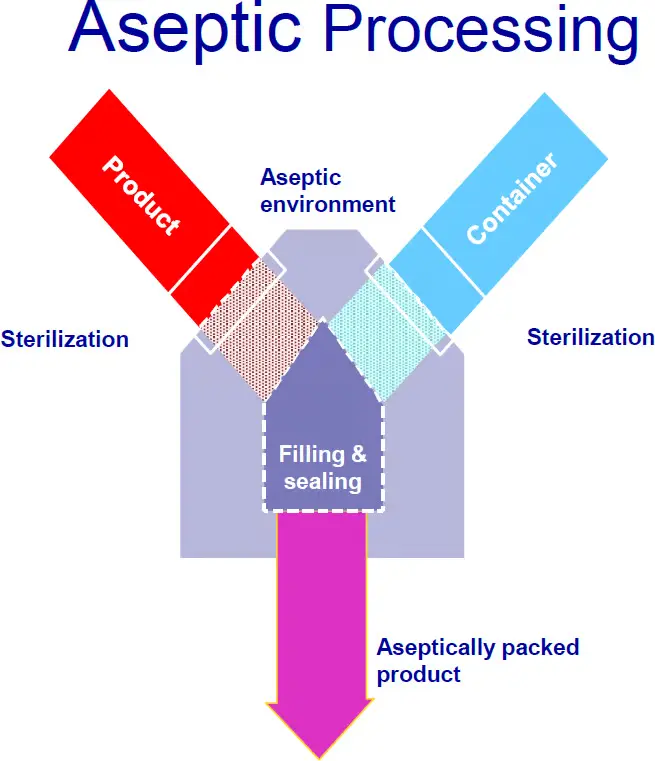
The importance of qualification services in the realm of aseptic processing cannot be overstated. At Thermosoft Technologies, a comprehensive approach is employed to ensure that both equipment and processes adhere to the industry’s stringent standards. The primary types of qualification services provided include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each of these qualifications plays a critical role in maintaining aseptic integrity.
Validations/Verifications
Pasteurization technologies
Explore the critical differences between Ultra-High Temperature (UHT) and High-Temperature Short Time (HTST) processing methods in the food industry. This comprehensive post discusses the benefits and applications of each method for enhancing food safety and quality. Learn how Thermosoft Technologies offers qualification and validation services for these processes, helping food manufacturers navigate compliance and operational efficiency. Discover insightful case studies illustrating the impact of effective system qualification on product safety and production efficiency.
Thermosoft Technologies provides a comprehensive array of qualification and validation services tailored specifically for Ultra High Temperature (UHT) and High Temperature Short Time (HTST) processing systems. These services are vital in ensuring that food processing equipment meets the stringent standards set forth by regulatory bodies, thereby safeguarding product quality and consumer safety. The qualification process typically involves several stages, including equipment validation, performance qualification, and ongoing monitoring.
Equipment validation is a critical first step, which assesses whether the UHT and HTST systems are installed correctly and function according to the manufacturer’s specifications. This involves rigorous testing under various operating conditions to ensure reliability and efficacy in pasteurization processes. Following this, the performance qualification phase verifies that the systems consistently operate within predetermined parameters, achieving the required temperature and time combinations necessary for effective microbial reduction.
Ongoing monitoring is an essential part of the validation continuum, allowing for real-time data collection and analysis. Thermosoft Technologies employs advanced methodologies to monitor system performance continuously, which aids in identifying any deviations from standard operating procedures. This proactive approach not only ensures compliance with health regulations but also mitigates risks associated with potential equipment failures.
Validation processes for UHT (Ultra-High Temperature) and HTST (High-Temperature Short Time) pasteurization are vital for ensuring the safety and quality of pasteurized products. The validation is typically delineated into three critical components, all of which contribute to maximizing the effectiveness of these thermal processes in eliminating pathogenic microorganisms.
The first component involves the qualification of pasteurization tanks regarding maximum temperature levels and temperature distribution. This stage is primarily achieved through a sterilization-in-place (SIP) procedure, which is designed to ensure that the tank reaches and maintains the necessary temperature throughout the entire system. By assessing how heat is distributed within the tank, manufacturers can verify that all areas reach the required temperature for the adequate time period, thereby ensuring complete sterilization of the product being processed.
The second part focuses on the validation of temperature/time profiles throughout the holding tube, a critical aspect of the UHT and HTST systems. During this evaluation, specific time-temperature combinations are tested to confirm that the product is sufficiently heated before it is packaged. This is essential in achieving the desired level of microbial inactivation. Utilizing calibrated thermocouples and data logging equipment, processors can monitor thermal histories to ensure that product thermalization consistently exceeds the defined parameters for safety and quality.
The third component of the validation process emphasizes the importance of continuous temperature monitoring across pipelines and holding tubes. Variations in temperature can indicate potential fouling or blockages that may compromise product safety. Regularly inspecting and monitoring these systems ensures that any irregularities are swiftly addressed, thereby guaranteeing the integrity of the pasteurization process.
The effectiveness of Ultra-High Temperature (UHT) and High-Temperature Short-Time (HTST) pasteurization processes is significantly influenced by microbial contamination. These preservation methods are widely employed in the food and beverage industry to extend shelf life and ensure product safety by eliminating pathogenic microorganisms that could compromise the quality of the finished products. However, the persistent threat posed by microbial organisms necessitates a thorough understanding of their behavior and impact during processing.
Microbial contamination can occur at various stages, from raw material handling to post-processing, potentially affecting the overall efficiency of UHT and HTST pasteurization. Gram-positive bacteria, such as Bacillus cereus, and various spores can withstand high-temperature processing, thereby posing a considerable challenge. If these microbes are not effectively destroyed, they may lead to spoilage or foodborne illnesses, highlighting the need for stringent controls throughout the pasteurization process.
Thermal mapping represents a critical component in validating the efficiency of UHT (Ultra-High Temperature) and HTST (High-Temperature Short-Time) pasteurization processes. This methodology serves to ascertain the thermal distribution within pasteurization systems, ensuring that food products achieve the requisite temperatures for the necessary duration to effectively reduce microbial loads. The primary objective of thermal mapping is to identify temperature variances throughout the pasteurization process, which may result from equipment design, product viscosity, or flow patterns. By placing temperature probes at strategic locations within the pasteurization unit, operators can generate detailed temperature profiles that inform on the performance and efficacy of the system.
The validation process for UHT (Ultra High Temperature) and HTST (High Temperature Short Time) pasteurization systems is crucial for ensuring product safety and quality. However, it presents a range of challenges that must be meticulously addressed. One of the primary challenges is maintaining consistent temperature profiles throughout the pasteurization process. Variations in temperature can lead to insufficient pathogen destruction, potentially allowing harmful microorganisms to survive, which compromises food safety.
Document compliance is another significant hurdle in the validation process. Regulatory agencies require detailed records of the pasteurization cycles, including time-temperature data, to ensure that processes adhere to established safety standards. Inconsistent or incomplete documentation can result in non-compliance, which may lead to fines, recalls, or other repercussions for manufacturers.
Accurate microbial testing also plays a critical role in validation. Laboratory methods must be reliable and robust in detecting any surviving pathogens or toxins that may have escaped the thermal process. However, logistical difficulties, such as sampling protocols and timely analysis, can hinder the effectiveness of microbial testing, potentially allowing subpar products to reach consumers.
To overcome these challenges, food processing facilities can implement several best practices. Regular calibration of temperature sensors and continuous monitoring systems can help maintain consistent temperature profiles during pasteurization. Furthermore, developing a comprehensive documentation strategy that includes automated record-keeping can ensure compliance with regulatory requirements. Conducting rigorous training programs for staff involved in microbial testing will enhance the reliability of results, ultimately supporting the validation process.
At TST, we offer advanced process authority and validation services in support of thermally processed shelf-stable food products. Our teams are industry experts and come well-equipped with industry-leading thermal validation equipment