Cooker Cooler
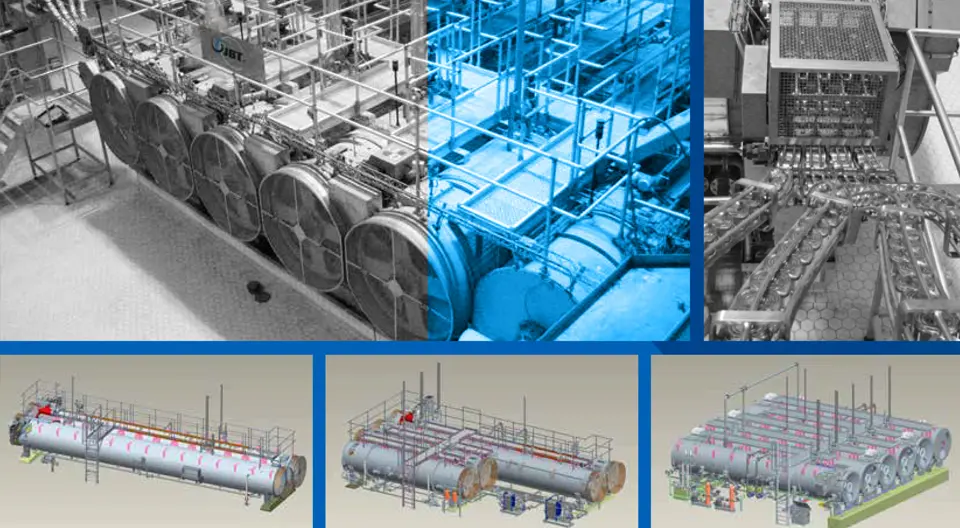
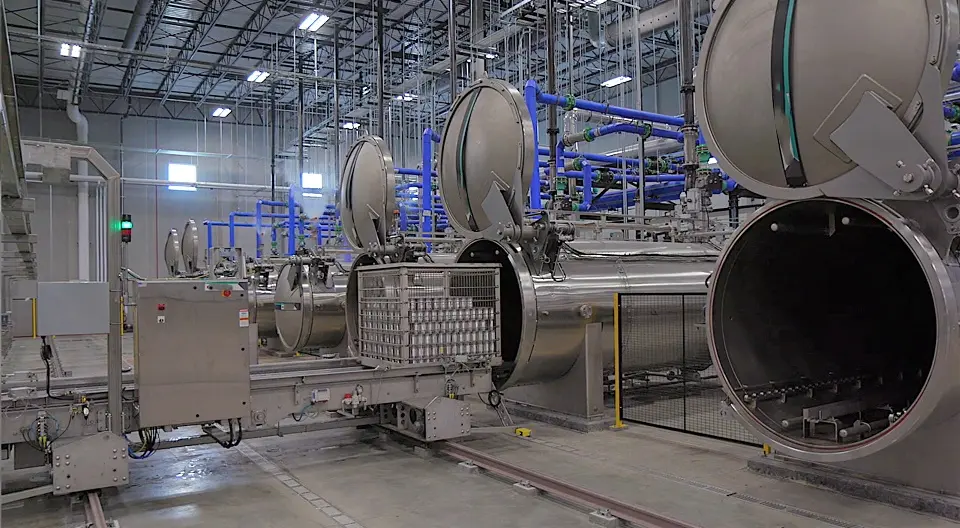
Complete Solutions for Cooker Cooler Qualification and Validation Cooker cooler qualification and validation often require tailored solutions to ensure complete adherence to safety and efficacy standards in food processing environments. TST provides a comprehensive suite of solutions aimed at addressing these specific qualification and validation needs. Each solution is designed to enhance operational efficiency while maintaining the integrity of products. One of the cornerstone offerings is temperature mapping software. This tool enables users to gather critical temperature data throughout various stages of the cooking and cooling processes. User-friendly and accurate, the software allows for real-time monitoring and reporting, identifying potential deviations in temperature control that could compromise food safety. The advantage of this software is its ability to facilitate compliance with regulatory standards, thus protecting your business from potential penalties. Another key solution is the integration of advanced data loggers. These devices serve to collect temperature and humidity data automatically throughout the cooking and cooling phases. By employing data loggers, facilities can minimize human error associated with manual readings while ensuring a reliable record for audits. Data loggers can also be configured to send alerts if thresholds are breached, enabling timely interventions that safeguard product quality. TST also offers meticulously crafted validation protocols tailored to suit various operational needs. These protocols guide businesses in conducting thorough checks on their cooker cooler systems, covering parameters such as thermal profiling and performance testing. By adhering to these protocols, organizations can ensure their systems are operating effectively, thereby mitigating risks related to food safety. In addition to these innovative solutions, TST shares success stories that highlight the effectiveness of their tools. Many clients have reported significant improvements in compliance rates and operational efficiencies after implementing TST’s recommended solutions. Thus, the range of services offered not only aids compliance but also enhances overall process efficacy in cooker cooler systems. Some of the products currently processed Fruit Ready meals Pastas Sauces Seafood Soups Nutritional drinks Infant formula (Evaporated) milk Cream Vegetables Vacuum packed corn Mushrooms Pharmaceuticals Meats Juices Rice based foods Congee Efficient Sterilization of Closed Cans The sterilization of closed cans is a crucial procedure in food processing, ensuring safety and extending shelf life. This process begins as cans enter the sterilizer directly from the closing machine, minimizing waiting time. Such efficiency is vital in a competitive market, as it reduces operational bottlenecks. Mechanics of the Sterilization Vessel A feed device plays a significant role in this operation, delivering the cans into the revolving reel of the first vessel efficiently. The interaction between this reel and a stationary spiral advances each can through the vessel, ensuring no can remains stagnant. The continuous spiraling motion, paired with the rotation of the container within the vessel, guarantees uniform heat distribution, enabling an even cook for every container. Consistent Treatment and Cooling One of the standout features of this system is that every can is treated individually, receiving the same thermal process regardless of its position in the sterilizer. After cooking, the cans are transferred to a water-filled cooling vessel where a similar process ensures they cool down efficiently. This method is designed to uphold the quality of the product while ensuring safety standards are met. Maximizing Efficiency with Continuous Rotary Pressure Sterilizers Continuous rotary pressure sterilizers are revolutionary machines designed to enhance the efficiency of cooking and cooling processes in food preservation. Unlike traditional methods, these sterilizers utilize a continuous operation model that allows for greater automation, ultimately optimizing production timelines in food manufacturing environments. The Benefits of Axial Agitation These sterilizers incorporate a unique design that allows the can to undergo a 3-phase movement cycle for each turn of the reel. This intermittent axial agitation not only facilitates convection heating but also results in significant time savings during the cooking and cooling stages. By swiftly and evenly distributing heat, continuous rotary pressure sterilizers ensure that food products are cooked thoroughly while minimizing the risk of spoilage. Enhancing Food Processing Efficiency One notable advantage of these machines is their ability to achieve high-temperature cooking for short periods of time, coupled with rapid cooling. This efficiency in processing leads to improved product quality and less energy consumption. As food manufacturers continue to seek methods for sustainable and economical production, continuous rotary pressure sterilizers stand out as a trustworthy solution that not only meets operational needs but also supports food safety standards. A Key to High Quality and Safety in Every Can In today’s competitive market, product uniformity is crucial for ensuring high quality and safety in food manufacturing. This blog post explores the importance of consistent processing methods and quality assurance practices that guarantee every product delivers the same taste, nutrients, and appearance. By implementing standardized thermal processes, manufacturers can maintain integrity, meet safety guidelines, and foster consumer trust. Learn how reliable product uniformity benefits both health-conscious consumers and brand reputation in the crowded market. Blog Food Technology In Container Sterilization Uncategorized Hydrostatic Retorts Complete Solutions for Hydrostatiс Retort Qualification and Validation Hydrostatic retorts are essential devices utilized primarily in the food processing and… Read More Smoke House Complete Solutions for Smoke House Qualification and Validation In the realm of food processing and preservation, smoke houses play a… Read More Cooker Cooler Complete Solutions for Cooker Cooler Qualification and Validation Cooker cooler qualification and validation often require tailored solutions to ensure complete… Read More Tunnel Pasteurization Complete Solutions for Qualification and Validation of Pasteurization Tunnels Pasteurization tunnels are key components in the food and beverage processing… Read More Retort Sterilization Comprehensive Solutions for Qualification and Validation in Retort Sterilization Retort sterilization is a fundamental process in the food processing industry,… Read More