
Why Thermal Process Validation is Crucial for Canned Food Manufacturers? Canned foods are a convenient and long-lasting option for consumers,...
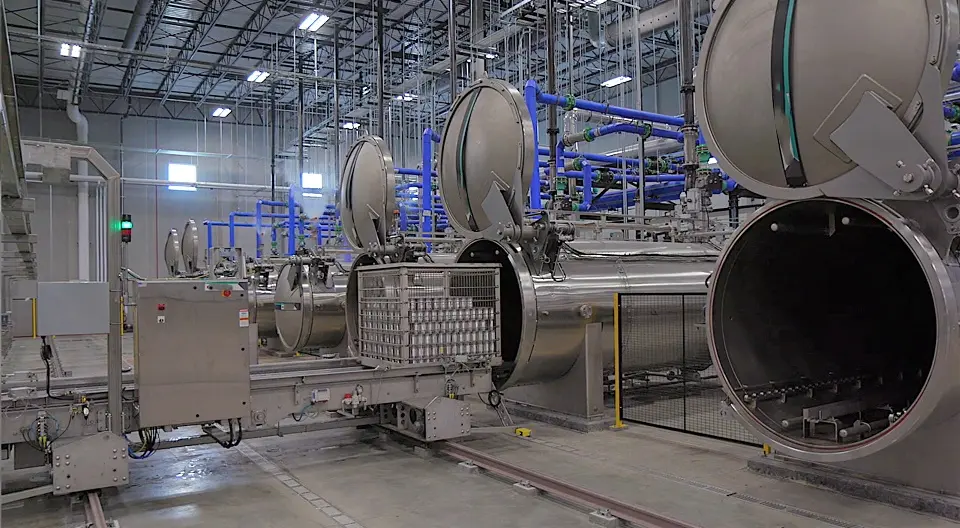
Retort sterilization is a fundamental process in the food processing industry, primarily designed to extend the shelf life of canned and packaged foods while ensuring safety for consumer consumption. This method employs high-temperature steam or water, effectively destroying pathogenic microorganisms that could cause foodborne illnesses. The importance of retort sterilization cannot be overstated, as it provides a reliable means to ensure food safety and quality throughout the supply chain.
At the core of retort systems lies the principle of pressure cooking, wherein food packages are subjected to elevated temperatures and pressures. The typical operation involves sealing food within a container and placing it into a specialized retort chamber. During the process, the chamber fills with steam or hot water, raising the temperature above 121 degrees Celsius (250 degrees Fahrenheit). This heat penetrates the food, leading to sterilization and the inactivation of pathogens. The efficacy of this process is further enhanced by the pressure, which allows for higher temperatures without the food boiling away, particularly beneficial for liquid-containing products.
There are various types of retorts used in the industry, including batch, continuous, and rotary retorts. Each type serves specific operational requirements, with batch retorts being ideal for smaller production volumes, while continuous and rotary systems cater to higher outputs. Ensuring the effectiveness of these systems necessitates rigorous qualification and validation protocols. Qualification processes align the equipment with regulatory industry standards, while validation focuses on demonstrating that the sterilization process reliably achieves the intended microbial reduction.
In summary, the realm of retort sterilization plays a pivotal role in food safety by effectively eliminating harmful microorganisms, thereby enhancing the overall integrity of food products. The meticulous nature of qualifying and validating retort systems is critical for maintaining consistent product quality and compliance within the food processing sector.
The qualification process for retort sterilization systems is a critical component in ensuring food safety and compliance with regulatory standards. This process typically consists of three main stages: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each stage serves a unique purpose, contributing to the overall validation of the retort system.
Installation Qualification (IQ) is the first phase, focusing on verifying that the retort is installed correctly according to manufacturer specifications. This step involves checking the physical installation, utility connections, and equipment calibration. It is essential to ensure that all components are configured properly, as any discrepancies can lead to significant operational issues later on.
The next phase, Operational Qualification (OQ), assesses whether the retort operates within specified limits across various operating conditions. This includes testing critical parameters such as temperature, pressure, and time, ensuring that the equipment functions correctly despite changes in the operational environment. OQ is crucial as it confirms the reliability and robustness of the system in terms of its operational capabilities.
Finally, Performance Qualification (PQ) involves demonstrating that the retort consistently achieves the desired sterilization results during actual production runs. This phase tests the system under real-world conditions, ensuring that it can meet the established criteria for ensuring product safety. During PQ, multiple batches should be tested to establish confidence in the system’s ongoing performance.
Documentation plays a pivotal role throughout this entire qualification process. Thorough and accurate records not only facilitate compliance with regulations but also provide critical evidence during audits. Best practices dictate that all steps taken during IQ, OQ, and PQ are meticulously documented, with deviations and corrective actions clearly noted. Maintaining such records aids in continuous improvement and ensures that the retort systems remain validated over time.
Effective sterilization is crucial in the food packaging industry, particularly when employing retort sterilization methods. A variety of validation techniques are employed to ensure the effectiveness of these processes, thus instilling confidence in both manufacturers and consumers. Among the most recognized methods is the use of biological indicators, which are essential for assessing the lethality of the sterilization process. These indicators contain specific microorganisms that are highly resistant to heat, serving as benchmarks for validating that the retort system achieves adequate sterilization levels.
Another critical method employed during validation is thermal mapping. This technique involves the systematic measurement of temperature throughout the retort system to ensure uniform heat distribution. By identifying cold spots and ensuring that every area of the container reaches the necessary temperature for sufficient time, thermal mapping helps in optimizing the retort cycles. Additionally, cycle verification is an indispensable practice that examines whether the established operational parameters are consistently met throughout the sterilization process.
Routine validation practices must also be part of the protocol, as they play a significant role in maintaining the efficiency and compliance of retort systems. Regular testing not only helps in identifying any equipment malfunctions, but also ensures adherence to the evolving regulatory and safety standards. Re-validation, particularly after any significant change in processing conditions, equipment upgrades, or maintenance activities, reinforces the assurance of a continued effective sterilization. By implementing these techniques, manufacturers can avoid potential pitfalls and guarantee that their products are safely processed and preserved.
TST provides a comprehensive suite of tailored solutions for the qualification and validation of retort sterilization systems, ensuring that food manufacturers meet the highest safety and quality standards. Understanding that each facility may have unique sterilization requirements, TST focuses on customizable services that cater to individual operational needs. This approach not only enhances compliance with regulatory standards but also optimizes productivity within food processing environments.
A critical component of TST’s service offerings is the thorough assessment of existing retort systems. By utilizing advanced diagnostic tools, TST evaluates the performance characteristics and identifies areas for enhancement, facilitating the development of targeted solutions. These assessments cover factors such as temperature distribution, pressure calibration, and sterilization cycle efficacy. Based on the findings, TST creates a tailored qualification plan that aligns precisely with the specific retort sterilization requirements of each client.
In addition to qualification services, TST offers robust validation protocols designed to ensure continuous adherence to operational standards. These include the design and implementation of rigorous testing procedures to validate the performance of the retort system throughout its lifecycle. TST also provides training sessions for operational staff, empowering them with the knowledge and skills necessary to effectively manage sterilization processes.
Notably, TST’s success can be evidenced through various case studies highlighting substantial improvements in operational efficiency and compliance reporting. Many food manufacturers have experienced a significant reduction in product recalls and enhanced traceability as a result of these tailored solutions. Customer testimonials further illustrate the positive impact of TST’s services in transforming retort sterilization practices, thus affirming its role as a leader in the field.

Why Thermal Process Validation is Crucial for Canned Food Manufacturers? Canned foods are a convenient and long-lasting option for consumers,...

Navigating FDA Regulations: FCE Registration and SID Filings It is widely known in the canning industry that the U.S. Food...

Thermal Process Validation – Hot Fill & Hold July 6, 2025 The Hot-Fill-Hold method is a crucial processing technique specifically...

Retort Temperature Distribution Study and Product Heat Penetration Study Thermal processing, specifically a technique known as commercial sterilization, is a...

Thermal Process Authority in India Thermosoft Technologies has established itself as a leading thermal process authority in India, known for...

How to Ensure a Smooth Food and Beverage Sterilization Process? Ensuring a smooth food and beverage sterilization process is critical...

Ultimate Challenge in Retort Process: Finding the Balance between Food Safety & Quality For low-acid foods packed in the hermetically...

In-Container Sterilization – Ensuring Commercial Sterilization Through Critical Factors as per USFDA Guidelines Introduction:The term “Commercial Sterilization” refers to a...

Thermal validation is a crucial aspect of food processing that involves determining the effectiveness of heat treatment methods, particularly in...
At TST, we offer advanced process authority and validation services in support of thermally processed shelf-stable food products. Our teams are industry experts and come well-equipped with industry-leading thermal validation equipment